|
|
Identification and verification of methylenetetrahydrofolate dehydrogenase 1-like protein as the binding target of natural product pseudolaric acid A
Haoqi Dong, Xinni Yang, Peiying Wang, Weiya Huang, Liang Zhang, Song Song, Jiangxin Liu
Natural Products and Bioprospecting. 2025, 15 (3): 21-21.
DOI: 10.1007/s13659-025-00502-1
Natural product pseudolaric acid A (PAA), the main bioactive component from Traditional Chinese Medicine Pseudolarix cortex (“tujingpi”), is a promising anticancer agent. However, its potential molecular targets are not clear and this hinders its development. In this study, chemical proteomics approaches including activity-based protein profiling (ABPP) and drug affinity responsive target stability (DARTS) technology, followed by quantitative proteomics, were combined to reveal the target of PAA. Target validation was performed by NMR techniques and surface plasmon resonance. Methylenetetrahydrofolate dehydrogenase 1-like (MTHFD1L) was identified and further confirmed to be the target of PAA. The direct interaction and binding mode between MTHFD1L and PAA were elaborated. PAA induced the accumulation of the reactive oxygen species (ROS) which mediates the antitumor effect. Transcriptome and network pharmacology analysis reveals the effects of PAA on the gene expressions of the associated pathways. Taken together, our findings proposed a new target that could be used for structure-based rational design and modifications of PAA.
References |
Related Articles |
Metrics
|
|
|
Structurally diverse polyketides and alkaloids produced by a plant-derived fungus Penicillium canescens L1
Wei-Ye Wu, Xun Wei, Qiong Liao, Yi-Fan Fu, Lei-Ming Wu, Lei Li, Shu-Qi Wu, Qing-Ren Lu, Fang-Yu Yuan, Dong Huang, Zhang-Hua Sun, Tao Yuan, Gui-Hua Tang
Natural Products and Bioprospecting. 2025, 15 (3): 22-22.
DOI: 10.1007/s13659-025-00503-0
A series of structurally diverse polyketides (1-3), sesterterpenoids (24 and 25), and alkaloids (26-34) were isolated from the fermentation of a plant-derived fungus Penicillium canescens L1 on solid rice medium. Among these secondary metabolites, penicanesols A-G (1-7) were new structures, which were elucidated by NMR, HR-ESI-MS, ECD calculation, and X-ray diffraction. Penicanesol A (1) represented a rare dimer derived from phthalan derivatives, characterized by a 5/6/6/6/5 heteropentacyclic core. The bioassay on the NCI-H1975 cell model showed that two compounds had good cytotoxic activities, and the most significant activate compound 13 had an IC50 value of 4.24±0.13 μM, more than the positive control drug (12.99±0.13 μM).
References |
Related Articles |
Metrics
|
|
|
Xylariaides A and B, novel cytochalasans with a unique 5/6/5/3 ring system from a soil fungus Xylaria sp. Y01
Yi-Yun Yuan, Yan Li, Wen-Yu Lu, Ai-Lin Liang, Jing Li, Wen-Xuan Wang
Natural Products and Bioprospecting. 2025, 15 (3): 23-23.
DOI: 10.1007/s13659-025-00507-w
Two new cytochalasans, xylariaides A (1) and B (2), were isolated and identified from a soil fungus Xylaria sp. Y01. Their structures were unambiguously determined by extensive spectroscopic methods including high resolution electrospray ionization mass spectrometry, ultraviolet radiation, infrared spectroscopy, and 1D/2D NMR, as well as in-depth quantum chemical calculations of gauge-including atomic orbital (GIAO) 13C NMR chemical shifts, electronic circular dichroism (ECD), and spin-spin coupling constants. The unprecedented core structure with a 5/6/5/3 fused tetracyclic ring system further enriches the scaffold types of cytochalasans.
References |
Related Articles |
Metrics
|
|
|
Activation of SIK1 by phanginin A regulates skeletal muscle glucose uptake by phosphorylating HADC4/5/7 and enhancing GLUT4 expression and translocation
Yu Shi, Xing-de Wu, Yanli Liu, Yu Shen, Hui Qu, Qin-Shi Zhao, Ying Leng, Suling Huang
Natural Products and Bioprospecting. 2025, 15 (3): 24-24.
DOI: 10.1007/s13659-025-00504-z
Salt-inducible kinase 1 (SIK1) participates in various physiological processes, yet its involvement in regulating skeletal muscle glucose uptake remains unclear. Previously, we showed that phanginin A, a natural compound isolated from Caesalpinia sappan Linn, activated SIK1 to suppress gluconeogenesis in hepatocytes. Here, we aimed to elucidate the effects of SIK1 on skeletal muscle glucose uptake by using phanginin A. The C2C12 myotubes were incubated with phanginin A and then glucose uptake, mRNA levels, membrane GLUT4 content, phosphorylation levels of proteins in SIK1/HDACs and Akt/AS160 signaling pathways were determined. Phanginin A significantly promoted glucose uptake, while the pan-SIK inhibitor or knocking down SIK1 expression abolished the promotion. Further exploration showed that phanginin A enhanced GLUT4 mRNA levels by increasing histone deacetylase (HDAC) 4/5 phosphorylation and MEF2a mRNA and protein level, and knocking down SIK1 blocked these effects. Additionally, phanginin A induced HDAC7 phosphorylation, upregulated the junction plakoglobin (JUP) expression and Akt/AS160 phosphorylation. Knocking down JUP or SIK1 both attenuated the phanginin A-induced Akt/AS160 signaling and glucose uptake, suggesting that activation of SIK1 by phanginin A inactivated HDAC7 to increase JUP expression and Akt/AS160 phosphorylation, led to upregulation of GLUT4 translocation and glucose uptake. In vivo study showed that phanginin A increased phosphorylation levels of SIK1, HDAC4/5/7, Akt/AS160, and gene expression of MEF2a, GLUT4 and JUP, accompanied by elevated membrane GLUT4 and glycogen content in gastrocnemius muscle of C57BL/6 J mice, indicating enhanced glucose utilization. These findings reveal a novel mechanism that SIK1 activation by phanginin A stimulates skeletal muscle glucose uptake through phosphorylating HADC4/5/7 and the subsequent enhancement of GLUT4 expression and translocation.
References |
Related Articles |
Metrics
|
|
|
Natural anticancer agents: prospection of medicinal and aromatic plants in modern chemoprevention and chemotherapy
Patricia Quintero-Rincón, Karina Caballero-Gallardo, Jesus Olivero-Verbel
Natural Products and Bioprospecting. 2025, 15 (3): 25-25.
DOI: 10.1007/s13659-025-00511-0
Natural products obtained from medicinal and aromatic plants are increasingly recognized as promising anticancer agents due to their structural richness, including terpene and flavonoid molecules, which induce apoptosis and modulate gene expression. These compounds offer an alternative to conventional treatments, often costly, which face challenges such as multidrug resistance. This review aims to provide a promising alternative approach to effectively control cancer by consolidating significant findings in identifying natural products and anticancer agent development from medicinal and aromatic plants. It synthesizes the findings of a comprehensive search of academic databases, such as PubMed and Springer, prioritizing articles published in recognized peer-reviewed journals that address the bioprospecting of medicinal and aromatic plants as anticancer agents. The review addresses the anticancer activities of plant extracts and essential oils, which were selected for their relevance to chemoprevention and chemotherapy. Compounds successfully used in cancer therapy include Docetaxel (an antimitotic agent), Etoposide VP-16 (an antimitotic agent and topoisomerase II inhibitor), Topotecan (a topoisomerase I inhibitor), Thymoquinone (a Reactive Oxygen Species-ROS inducer), and Phenethyl isothiocyanate (with multiple mechanisms). The review highlights natural products such as Hinokitiol, Mahanine, Hesperetin, Borneol, Carvacrol, Eugenol, Epigallocatechin gallate, and Capsaicin for their demonstrated efficacy against multiple cancer types, including breast, cervical, gastric, colorectal, pancreatic, lung, prostate, and skin cancer. Finally, it highlights the need for continued bioprospecting studies to identify novel natural products that can be successfully used in modern chemoprevention and chemotherapy.
References |
Related Articles |
Metrics
|
|
|
Phthalide mono- and dimers from the rhizomes of Angelica sinensis and their anti-inflammatory activities
Hongyan Wen, Sheng Li, Yu Zhang
Natural Products and Bioprospecting. 2025, 15 (3): 26-26.
DOI: 10.1007/s13659-025-00512-z
Three pairs of enantiomeric phthalide dimers, including two new ones, angesicolides A (1) and B (2), and a new phthalide monomer (3), were obtained from the rhizomes of Angelica sinensis. Their structures were established through spectroscopic methods, quantum calculations, and chiral HPLC analysis. Compounds 1 and 2 were [2+2] and [4+2] cycloadducts of phthalide monomers, and their hypothetical biogenetic origin was proposed. Compounds 2, (+)-2, (-)-2, 4, (+)-4, and (-)-4 exhibited significant inhibitory activity against NO production with IC50 values range from 1.23 to 5.55 μM.
References |
Related Articles |
Metrics
|
|
|
Inhibitory effects of corylin derived from aerial part of Pueraria lobata on melanin synthesis and potential applications in skin whitening and photoaging management
BoYoon Chang, SungYeon Kim
Natural Products and Bioprospecting. 2025, 15 (3): 27-27.
DOI: 10.1007/s13659-025-00509-8
Purpose This study aimed to investigate the potential of corylin, a bioactive compound isolated from the aerial part of Pueraria lobata, as a novel skin-whitening agent. Specifically, the research sought to evaluate its effects on melanin synthesis, understand its underlying mechanisms, and validate its efficacy in mitigating hyperpigmentation.
Methods Bioactive compound was isolated from Pueraria lobata through a systematic fractionation process involving activated carbon pigment removal, sequential solvent extraction, and resin-based chromatography. It was shown to inhibit melanin synthesis by targeting tyrosinase activation and modulating key signaling pathways. Its efficacy in reducing melanin production was validated through cellular assays and a UVB-stimulated 3D human skin model, highlighting its potential as a skin-whitening agent.
Results Through fractionation, the bioactive compound was identified as corylin, which reduced melanin content and tyrosinase activity without cytotoxicity, modulated signaling pathways to downregulate MITF and melanogenic enzymes, and inhibited α-glucosidase, disrupted glycosylation. In a UVB-stimulated 3D skin model, it effectively decreased melanin production, confirming its potential to mitigate hyperpigmentation.
Conclusion Corylin is a promising candidate for skin-whitening applications, effectively mitigating hyperpigmentation by targeting multiple stages of melanin synthesis, including enzymatic activity and regulatory pathways. Further clinical studies are needed to confirm its safety and therapeutic potential for dermatological use.
References |
Related Articles |
Metrics
|
|
|
Rational search for natural antimicrobial compounds: relevance of sesquiterpene lactones
Alejandro Recio-Balsells, Eugenia Rodriguez Ristau, Adriana Pacciaroni, Viviana Nicotra, Carina Casero, Manuela García
Natural Products and Bioprospecting. 2025, 15 (3): 28-28.
DOI: 10.1007/s13659-025-00513-y
Antimicrobial resistance is one of the most pressing global health challenges, as many pathogens are rapidly evolving to evade existing treatments. Despite this urgent need for new solutions, natural plant-derived compounds remain relatively underexplored in the development of antimicrobial drugs. This report highlights an innovative approach to discovering potent antimicrobial agents through bioguided fractionation of numerous plant species from the rich Argentinean flora. By systematically screening 60 species (over 177 extracts) for antimicrobial activity against representative strains of gram-positive and gram-negative bacteria, we identified promising bioactive compounds within the Asteraceae family—particularly sesquiterpene lactones from the Xanthium genus. Building on this basis, we synthesized semi-synthetic derivatives by chemically modifying plant sub-extracts, focusing on structures incorporating heteroatoms and/or heterocycles containing oxygen and nitrogen (important for the bioavailability and bioactivity that they are capable of providing). These modifications were evaluated for their potential to enhance antimicrobial efficacy against bacteria and Candida species, including resistant strains. Our findings suggest that tailoring natural metabolites from Xanthium and related Asteraceae species can significantly improve their antimicrobial properties. This strategy offers a promising pathway for the development of novel therapeutic agents to combat bacterial and fungal infections in an era of rising drug resistance.
References |
Related Articles |
Metrics
|
|
|
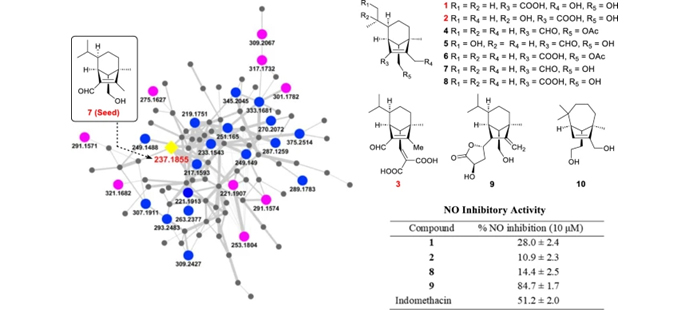
New sesquiterpenoids with anti-inflammatory effects from phytopathogenic fungus Bipolaris sorokiniana 11134
Qiang Yin, Jianying Han, Guixiang Yang, Zhijun Song, Keke Zou, Kangjie Lv, Zexu Lin, Lei Ma, Miaomiao Liu, Yunjiang Feng, Ronald J. Quinn, Tom Hsiang, Lixin Zhang, Xueting Liu, Guoliang Zhu, Jingyu Zhang
Natural Products and Bioprospecting. 2025, 15 (3): 29-29.
DOI: 10.1007/s13659-025-00508-9
Sesquiterpenoids represent a structurally diverse class of natural products widely recognized for their ecological significance and pharmacological potential, including antimicrobial, anti-inflammatory, and anticancer properties. As part of our efforts to explore bioactive secondary metabolites from phytopathogenic fungi, we conducted a molecular networking-based analysis of Bipolaris sorokiniana isolate BS11134, which was fermented on a rice medium. This analysis led to the identification of three new seco-sativene-type sesquiterpenoids (1-3) and seven known analogues (4-10), with the NMR data of compound 4 being reported for the first time. The structures of these compounds were elucidated using HR-ESI-MS and extensive spectroscopic data analysis. Notably, compound 9 significantly inhibited nitrous oxide expression in lipopolysaccharide (LPS)-treated RAW264.7 cells in vitro (inhibition rate: 84.7±1.7% at 10 μM), while compound 1 (10 μM) showed a weak inhibitory effect (inhibition rate=28.0±2.4%). Additionally, we proposed a biosynthetic pathway for these compounds. This study not only expands the chemical space of the helminthoporene class of molecules but also underscores the untapped potential of phytopathogenic fungi as promising sources of structurally unique and biologically active natural products.
References |
Related Articles |
Metrics
|
|
|
Therapeutic targeting of ocular diseases with emphasis on PI3K/Akt, and OPRL pathways by Hedera helix L. saponins: a new approach for the treatment of Pseudomonas aeruginosa-induced bacterial keratitis
Sherif A. Hamdy, Shymaa Hatem, Heba Elosaily, Abrar Gomaa Abd-Elfattah Hassan, Rana Elshimy, Ahmed H. Osman, Riham A. El-Shiekh
Natural Products and Bioprospecting. 2025, 15 (3): 30-30.
DOI: 10.1007/s13659-025-00514-x
Pseudomonas aeruginosa-induced bacterial keratitis is one of the most sight-threatening corneal infections associated with intense ocular inflammatory reactions that may lead to vision loss. Hence, this study investigated the efficacy of three nanocomposite chitosan-coated penetration enhancer vesicles (PEVs) to augment the ocular delivery of saponin(s), α-hederin (PEVI), hederacoside C (PEVII), or both (PEVIII) for treatment of Pseudomonas keratitis and its induced inflammatory response. The three formulations were prepared using the ethanol injection method and comprehensively characterized. In vitro, the antibacterial activity of the three formulations against P. aeruginosa was evaluated using agar well-diffusion method, pyocyanin production inhibition, and swarming and twitching motility inhibition assays. The therapeutic effect of the three formulations has been investigated in P. aeruginosa keratitis by gross lesion monitoring, determination of bacterial bioburden, biochemical markers, histopathological examination, and scoring after 7 days of topical treatment. Data revealed that PEVI, PEVII, and PEVIII nanocomposites showed particle size in the nanometer range, high entrapment efficiency, good stability, and sustained release of the saponins throughout 24 h. Among them, PEVIII exhibited notably strong in vitro antipseudomonal activity. Additionally, animals treated topically with PEVIII showed an appreciable gross lesion reduction, corneal tissue improvement, and formidable bacterial load reduction compared with untreated and gentamicin sulfate eye (GENTAWISE®) ointment-treated groups. Moreover, PEVIII treatment showed the most significant reduction in TNF-α, NF-κB, ROS levels, and OPRL virulence gene expression while enhancing PI3K/Akt activation. Therefore, this study offers PEVIII as a promising treatment for P. aeruginosa keratitis.
References |
Related Articles |
Metrics
|
|
 New sesquiterpenoids with anti-inflammatory effects from phytopathogenic
New sesquiterpenoids with anti-inflammatory effects from phytopathogenic
 Natural anticancer agents: prospection of medicinal and aromatic plants
Natural anticancer agents: prospection of medicinal and aromatic plants
 Xylariaides A and B, novel cytochalasans with a unique 5/6/5/3 ring system
Xylariaides A and B, novel cytochalasans with a unique 5/6/5/3 ring system
 Structurally diverse polyketides and alkaloids produced by a plant-derived fungus
Structurally diverse polyketides and alkaloids produced by a plant-derived fungus




